8 Biotechnology- Biology for Human Welfare
8.7 Vaccines
Vaccines
Dr V Malathi
Vaccines are biological preparations that are intended to produce immunity against particular illnesses. They stimulate the immune system to identify and combat the disease in the future by introducing antigens that are weakened, inactive, or fragments of a pathogen—into the body.
Type of Vaccines
Live Attenuated Vaccines
This type of vaccine contain a weakened (attenuated) form of the live pathogen. Though the pathogen is live it is altered so that it cannot cause disease in healthy individuals. Examples of such vaccines are Measles, Mumps, and Rubella (MMR) vaccine,Varicella (chickenpox) vaccine, Oral Polio Vaccine (OPV), Yellow Fever vaccine. These live attenuated vaccines are strong and long lasting. However these are not suitable for people with weakened immune systems and requires careful storage (cold chain).
Inactivated Vaccines
These vaccines are produced using a killed (inactivated) version of the pathogen. Examples:Inactivated Polio Vaccine (IPV),Hepatitis A vaccine ,Rabies vaccine. These vaccines are safer for people with weakened immune systems but usually requires multiple doses and booster shots for long-term protection.
Subunit, Recombinant, Polysaccharide, and Conjugate Vaccines
These vaccines are produced using specific pieces of the pathogen (like protein, sugar, or capsid). Examples: Hepatitis B vaccine,Human Papillomavirus (HPV) vaccine,Pneumococcal vaccine,Meningococcal vaccine. These vaccines have a targeted immune response and usually exhibit fewer side effects. These vaccines also require multiple doses for complete protection.
Toxoid Vaccines
Toxoid vaccines use inactivated toxins (toxoids) produced by bacteria, which cause disease symptoms. Examples:Tetanus vaccine ,Diphtheria vaccine. These provide immunity against toxins and not against the pathogen itself. For sustained immunity booster doses are required.
mRNA Vaccines
This type of vaccines introduce the messenger RNA (mRNA) into cells, instructing them to produce a protein that triggers an immune response.Examples: Pfizer-BioNTech COVID-19 vaccine, Moderna COVID-19 vaccine.These are advantageous as no live virus is involved but require cold storage
Viral Vector Vaccines
This type of vaccines use a harmless virus (vector) to deliver genetic material from the target pathogen. Examples: AstraZeneca COVID-19 vaccine,Johnson & Johnson COVID-19 vaccine. These vaccines elicit a strong immune response but a pre-existing immunity to the vector may reduce effectiveness of the vaccine
DNA Vaccines (Emerging)
This type of vaccines use DNA plasmids to introduce genetic material coding for antigens.Examples include some experimental vaccines like Zydus Cadila’s COVID-19 vaccine. These vaccines are stable and still under extensive study.
Essential steps in vaccine production using Biotechnology

“Vaccines” by Katie L. Flanagan, Emma Best, Nigel W. Crawford, Michelle Giles, Archana Koirala, Kristine Macartney, Fiona Russell, Benjamin W. Teh, and Sophie CH Wen, on behalf of the Australasian Society for Infectious Diseases (ASID) Vaccination Special Interest group (VACSIG), creativecommons.org via wikimedia commons is licensed under CC BY 4.0
- Identifying the Antigen and Pathogen : To find the elements that can trigger a potent immune response, scientists investigate the target pathogen (bacteria, virus, or toxin).
Common targets include: surface proteins, such as the SARS-CoV-2 spike protein. Toxins from germs such as diphtheria or tetanus that have been inactivated. - Choosing a Production Platform :
a) Recombinant DNA Technology: The method involves identification of the genes encoding the target antigen. Plasmids (small, circular DNA) are used to introduce these genes into a host organism, such as bacteria, yeast, or mammalian cells. The antigen is produced in huge quantities by cultivating the host organism in bioreactors. For instance, the Hepatitis B vaccine is made by generating the Hepatitis B surface antigen (HBsAg) from yeast cells.
b) mRNA Technology: in this method the target antigen’s mRNA is produced in a laboratory. Lipid nanoparticles are used to transport the mRNA into human cells.Example: Pfizer-BioNTech and Moderna COVID-19 vaccines.
c) Viral Vector Technology: A harmless virus, such as an adenovirus, is genetically altered to contain the targe antigen’s genetic material. The altered virus undergoes purification and culture. For instance, the COVID-19 vaccinations from AstraZeneca and Johnson & Johnson.
d) Protein Subunit Vaccines: In this method recombinant technologies or cell cultures are used to manufacture certain pathogen proteins. After purification, adjuvants are added to these proteins to strengthen the immune response. For instance, the COVID-19 vaccination Novavax.
3. Cultivation and Fermentation : In regulated bioreactors, large-scale cultures of host cells—such as bacteria, yeast, or mammalian cells—are cultivated. The viral particles or antigen are captured, purified, and expressed.
4. Purification : Methods like chromatography and centrifugation are used to extract and purify the antigens or other vaccine components.This guarantees that there are no pollutants or impurities in the finished product.
5. Formulation : Stabilizers, preservatives, and adjuvants—substances that boost the immune response—are combined with the purified antigen. Certain vaccinations, like mRNA vaccines, are delivered by encapsulation in lipid nanoparticles.
6.Quality Control and Testing : Vaccines are put through extensive testing for safety and effectiveness (in clinical studies, animal models, and in vitro). The vaccines are checked for stability ,potency and batch-to-batch uniformity.
7. Packaging and Distribution : The vaccine is sterilely packed in syringes or vials. To preserve vaccine integrity, distribution adheres to stringent handling and temperature guidelines. or example cold chain requirements for mRNA vaccines).
Vaccine Components
| Component | Description |
|
Antigen
|
the vaccine’s active ingredient that triggers an immunological reaction.
|
|
Adjuvants
|
Component that increases the efficacy of the vaccinations. Adjuvants based on aluminum are used in most vaccinations. They aid the immune response by causing a variety of inflammatory substances to be produced at the injection site. The kidneys eliminate aluminum from the body through urine.
|
|
Preservatives
|
Preservatives prevent a vaccine from becoming unintentionally contaminated. 2-phenoxyethanol is the most widely used preservative and can also be found in a variety of cosmetics, baby care products, and ear and eye drops. Thimerosal, a preservative containing mercury that is used in multi-dose vaccines to stop pathogenic germs or fungi from contaminating them, may cause clients to worry. |
|
Stabilizers
|
Gelatin and other stabilizers prevent the vaccine’s constituents from separating and inhibit chemical reactions. Lactose, potassium, salt, and amino acids—the building blocks of proteins—are further stabilizers.
|
|
Buffers
|
When modest amounts of an acid or an alkali are introduced to a buffer solution, the pH of the solution does not change. Buffers maintain the vaccine’s pH close to that of the body. The buffer is frequently a salt.
|
|
Adjusting tonicity
|
A salt may be added to the vaccine to maintain its isotonicity and lessen local responses. Sodium chloride, or regular table salt is frequently used for this purpose
|
|
Surfactants and emulsifiers
|
These substances have a detergent-like effect. Polysorbate 80, also known as Tween®, is a surfactant that is frequently employed. Oleic acid, an omega fatty acid, and sorbitol, a sugar alcohol, are used to make this. Foods like ice cream frequently include polysorbate.
|
|
Formaldehyde
|
The live germ or toxin used in some vaccines is detoxified or rendered inactive by formaldehyde. Most of it is eliminated via the purifying procedure. It is significant to remember that all human bodies contain trace levels of formaldehyde, which is necessary for DNA synthesis. Formaldehyde degrades rapidly in the body and in the environment. Furthermore, a baby’s body contains roughly ten times as much formaldehyde as a vaccine does.
|
|
Antibiotics
|
Antibiotics are included in some vaccines to stop bacteria from growing while the vaccine is being stored.
|
Vaccine components table adapted from Vaccine Practice for Health Professionals: 1st Canadian Edition
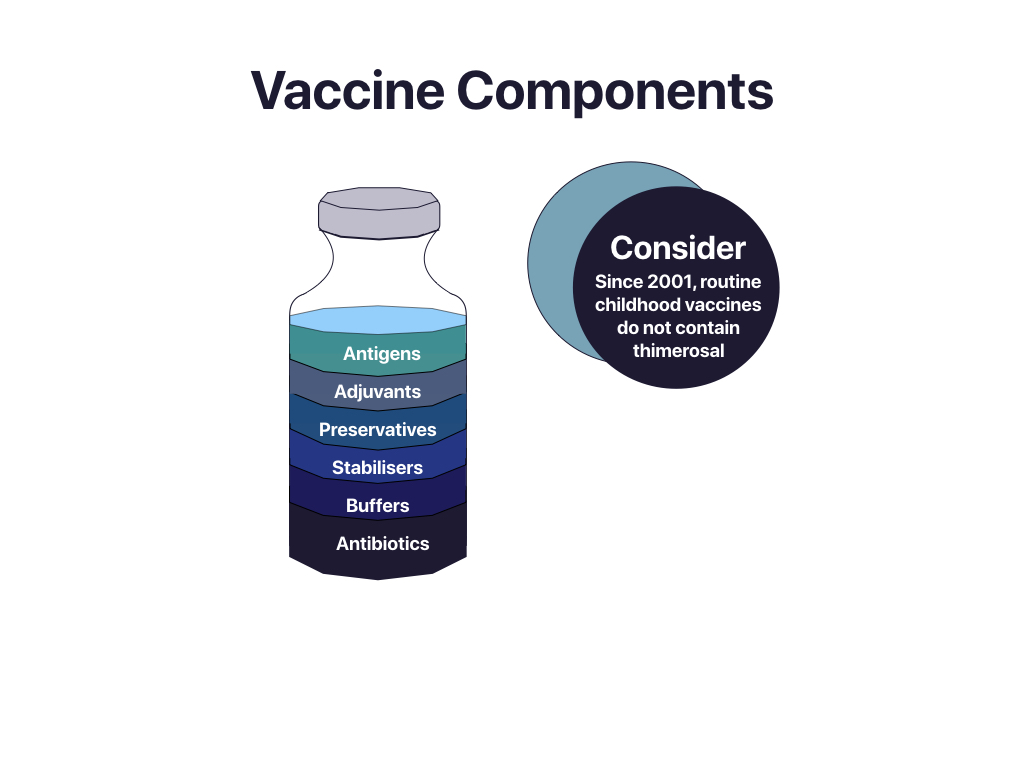
“Vaccines components” by Oona St-Amant; Jennifer Lapum; Vinita Dubey; Karen Beckermann; Che-Sheu Huang; Carly Weeks; Kate Leslie; and Kim is licensed under CC BY-SA 4.0
Test your understanding

