8 Biotechnology- Biology for Human Welfare
8.6 Animal Tissue Culture
Animal Cell culture
Dr V Malathi
The process of cultivating animal cells, tissues, or organs outside of the body in a regulated, artificial environment is known as animal tissue culture. This procedure has several uses in research, medicine, and biotechnology and allows the controlled study of cell behavior, biology, and function.Connective tissues including fibroblasts, skeletal, cardiac, and smooth muscle, epithelial tissues, neuronal cells, endocrine cells, and numerous tumor cell types are among the various cell types that may currently be cultivated in culture.
For in vitro growth of cells, the culture conditions may not mimic in vivo conditions with respect to temperature, pH, CO2, O2, osmolality, and nutrition. In addition, the cultured cells require sterile conditions along with a
steady supply of nutrients for growth and sophisticated incubation conditions
“Image of cell culture dish” is in the Public Domain, CC0
First animal cell culturing on Industrial scale
First animal cell culture was performed at an industrial scale in 1950s .The polio epidemic in 1940s and 1950s and the accompanying requirement for viral vaccines necessitated the the need for cell cultures on a large scale . The polio vaccine from a deactivated virus became one of the first commercial products developed from cultured animal cells
Applications of Animal cell culture
Animal cell cultures provided a model system for various research like:
- The study of basic cell biology, cell cycle mechanisms, specialized cell function, cell to cell and cell – matrix
interactions. - Toxicity testing to study the effects of new drugs.
- Gene therapy for replacing nonfunctional genes with functional gene-carrying cells.
- The characterization of cancer cells, the role of various chemicals, viruses, and radiation in cancer cells.
- Production of vaccines, mono clonal antibodies, and pharmaceutical drugs.
- Production of viruses for use in vaccine production (e.g., chicken pox, polio, rabies, hepatitis B, and measles).
- For manufacturing biological therapeutics such as hormones, antibodies, interferons, clotting factors, and
vaccines
Culture media
One of the most important factors in animal cell culture is the medium composition. In vitro growth and maintenance of animal cells require appropriate nutritional, hormonal, and stromal factors that resemble their in vivo condition as closely as possible.
The other important factors include : the medium in which the cells are surrounded, the substratum upon which the cells grow, temperature, oxygen and carbon dioxide concentration, pH, and osmolality. In addition, the cell requires chemical substances that cannot be synthesized by the cells themselves.
Any successful medium is composed of isotonic, low-molecular-weight compounds known as basal medium and provides inorganic salts, an energy source, amino acids, and various supplements
Basic components in culture media
The 10 basic components that make up most of the animal cell culture media are as follows:
- inorganic salts (Calcium, Magnesium , Sodium and Potassium)
- nitrogen source (amino acids),
- energy sources (glucose, fructose),
- vitamins, fat and fat soluble component (fatty acids, cholesterols),
- nucleic acid precursors,
- growth factors and hormones,
- antibiotics,
- pH and buffering systems, and
- oxygen & carbon dioxide concentrations.
Complete formulation of media that supports growth and maintenance of a mammalian cell culture is very complex. The nutritional requirements of cells can vary at different stages of the culture cycle. Different cell types have highly specific requirements, and the most suitable medium for each cell type must be determined experimentally
Types of Media
Animal cell culture media may be classified into two categories: as (1) natural media and (2) artificial media.
• Natural media
Natural media consist of naturally occurring biological fluids sufficient for the growth and proliferation of animals cells and tissues.
These are of the following three types:
1. Coagulant or clots: Plasma separated from heparinized blood from chickens or other animals is commercially available in the form of liquid plasma.
2. Biological fluids: This includes body fluids such as plasma, serum lymph, amniotic fluid, pleural fluid, insect hemolymph, and fetal calf serum. These fluids are used as cell culture media after testing for toxicity and sterility.
3. Tissue extract: Extracts of liver, spleen, bone marrow, and leukocytes are used as cell culture media. Chicken embryo extract is the most common tissue extract used in some culture media
- Artificial media
This media contains partly or fully defined components that are prepared artificially by adding several nutrients (organic and inorganic). It contains a balanced salt solution with specific pH and osmotic pressure designed for immediate survival of cells. Artificial media supplemented with serum or with suitable formulations of organic
compounds supports prolonged survival of the cell culture.
The artificial media may be grouped into the following four classes:
• serum-containing media,
• serum-free media,
• chemically defined media, and
• protein-free media
Serum- Containing media
Serum is the clear yellowish fluid obtained after fibrin and cells are removed from blood .It is an undefined media supplement of extremely complex mixture of small and large molecules and contains amino acids, growth factors, vitamins, proteins, hormones, lipids, and minerals, among other components .
Advantages of serum in cell culture medium
1. It has basic nutrients present either in soluble or in protein-bound form.
2. It provides several hormones such as insulin and transferrin. Insulin is essential for the growth of nearly all cells in culture and transferrin acts as an iron binder.
3. It contains numerous growth factors such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-B), epidermal growth factor (EGF), and chondronectin. These factors stimulate cell growth and support specialized functions of cells.
4. It supplies protein, which helps in the attachment of cells to the culture surface (e.g., fibronectin).
5. It provides binding proteins such as albumin and transferrin, which helps transport molecules in cells.
6. It provides minerals such as Ca, Mg, Fe, K,Na, Zn, etc., which promote cell attachment.
7. It increases the viscosity of the medium, which provides protection against mechanical damage during agitation and aeration of suspension cultures.
8. It provides appropriate osmotic pressure.
Disadvantages of serum-containing medium
- Expensive: Fetal calf serum is expensive and difficult to obtain in large quantities.
- Variation: Batch-to-batch variation occurs in serum, and there is no uniformity in composition of serum. This can affect growth and yields and can give inconsistent results.
- Contamination: Serum medium carries a high risk of contamination with virus, fungi, and mycoplasma
- Cytotoxic and inhibiting factors: The serum itself may be cytotoxic and may contain inhibiting factors, which in turn may inhibit cultured cell growth and proliferation. The enzyme polyamine oxidase in serum reacts with polyamines such as spermine and spermidine to form cytotoxic polyamino-aldehyde.
- Downstream processing: The presence of serum in culture media may interfere with isolation and purification of culture products. Additional steps may be required to isolate cell culture products.
Serum-free media
The use of serum in culture media presents a safety hazard and source of unwanted contamination for the production of biopharmaceuticals. As a number of cell lines can be grown in serum-free media supplemented with certain
components of bovine fetal serum, the development of this type of medium with a defined composition has intensified in the last few decades.
Eagle (1959) developed a “minimal essential medium” composed of balanced salts, glucose, amino acids, and vitamins.
Advantages of serum-free culture media
1. Serum-free media are simplified, and the composition is better defined.
2. They can be designed specifically for a cell type. It is possible to create different media and to switch
from growth-enhancing media to differentiation inducing media by altering the combination and types of growth factors and inducers.
3. They decrease variability from batch to batch and improve reproduction between cultures.
4. Downstream processing of products from cell cultures in serum-free media is easier.
5. They reduce the risk of microbial contamination (mycoplasma, viruses, and prions).
6. Serum-free media are easily available and ready to use. They are also cost-effective when compared
with serum-containing media.
Disadvantages of serum-free media
1. Growth rate and saturation density attained are lower than those compared to serum-containing media.
2. Serum-free media prove to be more expensive as supplementing with hormone and growth factors
increases the cost enormously.
3. Different media are required for different cell types as each species has its own characteristic requirements.
4. Critical control of pH and temperature and ultra purity of reagent and water are required as compared to
serum-containing media
Fetal bovine serum (FBS)
FBS supplemented media are commonly used in animal cell cultures. FBS is harvested from bovine fetuses taken from pregnant cows during slaughter. The common method of harvesting the fetus is by cardiac puncture without
any anesthesia. This practice of harvesting FBS is inhumane as it exposes the fetus to pain and/or discomfort.
In addition to moral concerns, numerous scientific and technical problems exist with regard to the use of FBS in cell culture. Efforts are now being made to reduce the use of FBS and replace it with synthetic alternatives
Types of Animal Tissue Culture
Primary Culture
In this type of culturing , cells are directly isolated from tissues by mechanical or chemical disintegration or by enzymatic digestion and grown in culture. They represents the condition that is most similar to the in vivo (natural) state. These cells have limited lifespan in culture. These cells are induced to grow in suitable glass or plastic containers with complex media. These cultures usually have a low growth rate and are heterogeneous however, they are still preferred over cell lines as these are more representative of the cell types in the tissues from which they are derived.
The morphological structure of cells in culture is of various types:
•Epithelium type, which are polygonal in shape and appear flattened as they are attached to a substrate and form a continuous thin layer (i.e., monolayer on solid surfaces);
• Epitheloid type, which have a round outline and do not form sheets like epithelial cells and do not attach to the substrate;
• Fibroblast type, which are angular in shape and elongated and form an open network of cells rather than tightly packed cells, are bipolar or multipolar, and attach to the substrate; and
• Connective tissue type, which are derived from fibrous tissue, cartilage, and bone, and are characterized by a large amount of fibrous and amorphous extracellular materials

“Fibroblasts” by Subtle Guest derivative work: Mfigueiredo, Creative commons .org via wiki media commons is licensed under CC BY 2.5
Types of primary cell culture
The primary cell culture can also be divided into two types.
Anchorage-dependent/adherent cells
These cells require a stable nontoxic and biologically inert surface for attachment and growth and are difficult to grow as cell suspensions. Mouse fibroblast STO cells are anchorage cells.
Anchorage-independent/suspension cells
These cells do not require a solid surface for attachment or growth. Cells can be grown continuously in liquid media. The source of cells is the governing factor for suspension cells.Blood cells are vascular in nature and are suspended in plasma and these cells can be very easily established in suspension cultures
Secondary cell culture
When primary cell cultures are passaged or subcultured and grown for a long period of time in fresh medium, they form secondary cultures. These are long lasting (unlike cells of primary cell cultures) due to the availability of fresh nutrients at regular intervals. The passaging or subculturing is carried out by enzymatic digestion of adherent cells. This is followed by washing and re-suspending of the required amount of cells in appropriate volumes of growth media.
Secondary cell cultures are preferred as these are easy to grow and are readily available.They have been useful in virological, immunological, and toxicological research
Cell line
The primary culture, when subcultured, becomes a cell line or cell strain that can be finite or continuous, depending on its lifespan in culture. They are grouped into two types on the basis of the lifespan of the culture.
Finite cell lines
Cell lines with a limited number of cell generations and growth are called finite cell lines. The cells are
slow growing (24 -96 hours). These cells are characterized by anchorage dependence and density limitation
Indefinite cell lines
Cell lines obtained from in vitro transformed cell lines or cancerous cells are indefinite cell lines and can
be grown in monolayer or suspension form. These cells divide rapidly with a generation time of 12-14 hours and have a potential to be subcultured indefinitely. The cell lines may exhibit aneuploidy or heteroploidy due to an altered chromosome number. Immortalized cell lines are transformed cells with altered growth properties.
HeLa cells are an example of an immortal cell line. These are human epithelial cells obtained from fatal cervical carcinoma transformed by human papilloma virus 18 (HPV18). Indefinite cell lines are easy to manipulate and maintain. However, these cell lines have a tendency to change over a period of time.
Organ culture
Whole organs from embryos or partial adult organs are used to initiate organ culture in vitro. These cells in the organ culture maintain their differentiated character, their functional activity, and also retain their in vivo architecture. They do not grow rapidly, and cell proliferation is limited to the periphery of the explant. As these cultures cannot be propagated for long periods, a fresh explanation is required for every experiment this leads to inter experimental variation in terms of reproducibility and homogeneity.
Organ culture is useful for studying functional properties of cells (production of hormones) and for examining the effects of external agents (such as drugs and other micro or macro molecules) .
Explant culture
Fragments exercised from animal tissue may be maintained in a number of different ways. The tissue adheres to the surface. This is aided by an extracellular matrix (ECM) constituent, such as collagen or a plasma clot, and
it can even happen spontaneously. This gives rise to cells migrating from the periphery of the explant. This culture is known as a primary explant, and migrating cells are known as outgrowth. This has been used to analyze the growth characteristics of cancer
Steps in Animal Tissue Culturing
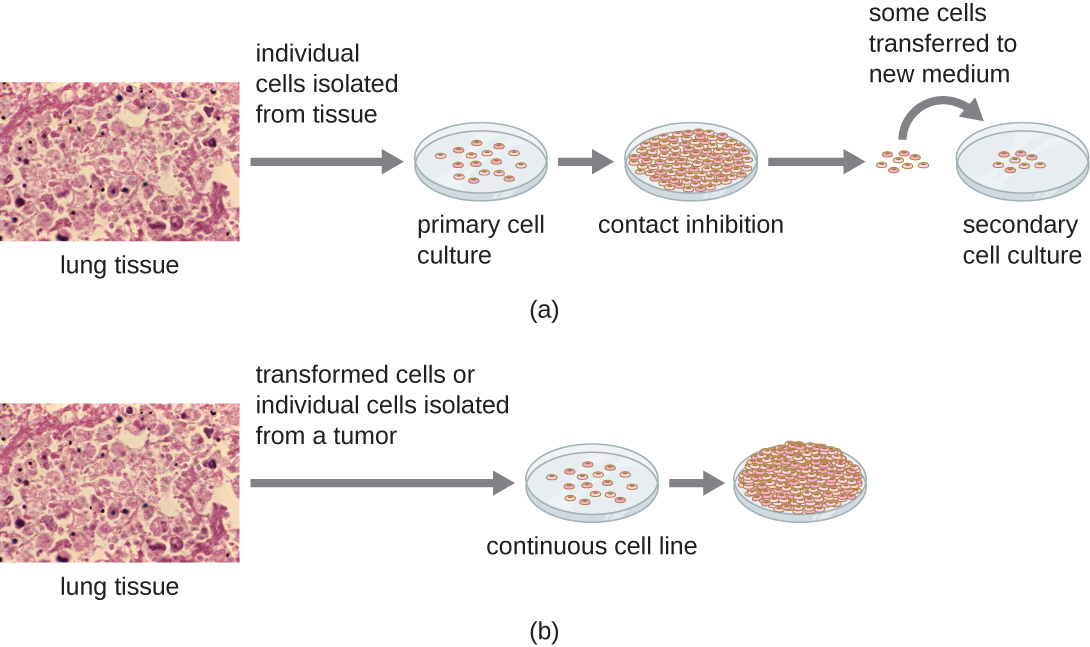
“a.Primary cell Culture and b.Continuous cell culture” by micrographs”: modification of work by Centers for Disease Control and Prevention) is licensed under CC BY 4.0
- Preparation of Explant : Sterile surgical techniques are utilized to remove tissue from an animal. Common sources include malignancies, the liver, kidneys, and skin.
-
Tissue Disintegration : The following methods are used to separate tissue into individual cells or smaller pieces. Mechanical techniques like grinding or cutting are used . Collagenase and trypsin are examples of enzymes that aid in enzymatic digestion.
- Preparing the Culture Medium : Essential nutrients, vitamins, and salts are included in nutrient-rich medium (such as DMEM and RPMI-1640). serum for growth factors, such as fetal bovine serum. Antifungals and antibiotics are added to stop contamination.
- Seeding : The medium is added to culture dishes, flasks, or bioreactors containing cells or tissues.
- Incubation : Cultures are preserved under particular circumstances: The usual temperature is 37°C.
CO₂ Levels: 5–10% CO₂ to keep the pH in check. Humidity: Keeps the medium from drying out. - Passaging (subculturing) : To prevent overpopulation, cells are moved to new media as they attain confluence.
- Cryopreservation (Optional) : For long term use cells are stored in liquid nitrogen (-196°C). This is achieved by using cryoprotectants like Dimethyl Sulphoxide (DMSO) and this process is referred as Cryopreservation
Listen to the Wikipedia audio article on Cell culture

