Main Body
Self-Assessment Activity and Responses
Activity 1A:
|
Thermal effect/change |
Name of thermal method |
|
Weight |
TG |
|
Dimension |
TMA |
|
Energy (difference in temperature) |
DTA |
|
Acoustic property |
Thermoacoustimery |
|
Optical property |
Thermopotometry |
|
Electrical conductivity |
Electrothermal Analysis |
|
Magnetic property |
Thermomagnetometry |
Activity 1B:
|
Technique |
Quantity Measured |
|
1)DSC |
Heat and temperature of transition and reactions |
|
2)DTA |
Temperature of transitions and reactions. |
|
3)EGA |
Amount of gaseous products of thermally induced reactions. |
|
4)TG |
Weight change |
Activity 1C:
|
Phenomenon |
Exothermic |
Endothermic |
|
Adsorption |
Y |
|
|
Desorption |
|
Y |
|
Fusion (melting) |
|
Y |
|
Vaporization |
|
Y |
|
Decomposition |
Y |
Y |
|
Dehydration |
|
Y |
Activity 1D:
|
Phenomenon |
Weight gain |
Weight loss |
Endothermic |
Exothermic |
|
Melting |
|
|
Y |
|
|
Adsorption of gas |
Y |
|
|
Y |
|
Desorption of gas |
|
Y |
|
Y |
|
Vaporisation |
|
Y |
Y |
|
|
Dehydration |
|
Y |
Y |
|
|
Decomposition |
|
Y |
Y |
Y |
|
Sublimation |
|
Y |
Y |
|
Activity 2A:
|
Phenomenon |
Physical |
Chemical |
|
Adsorption |
Y |
|
|
Dehydration |
|
Y |
|
Desorption |
Y |
|
|
Fusion (melting) |
Y |
|
|
Chemisorption |
|
Y |
|
Vaporization |
Y |
|
|
Decomposition |
|
Y |
|
Redox reactions |
|
Y |
|
Reduction in gaseous atmosphere |
|
Y |
Activity 2B:
a. The part of the TG curve where the mass is essentially constant – Plateau AB
b. The temperature at which cumulative mass change reaches a magnitude
that the thermobalance can detect -Point B
c. The temperature at which the cumulative mass change reaches a maximum-Point C
Activity 2C:
|
Phenomenon |
Type |
|
Sublimation |
Y |
|
Adsorption of gas |
X |
|
Desorption of gas |
Y |
|
Vaporisation |
Y |
|
Dehydration |
Y |
|
Decomposition |
Y |
|
Melting |
None |
Activity 2D:
A derivative thermogravimetric (DTG) curve represents a plot of mass of sample, as a function of temperature. T/F
A point of inflection on a thermogravimetric (TG) curve will correspond to a minimum on the DTG curve for a given chemical decomposition.T/F
A ‘plateau’ on a TG curve will not necessarily correspond to a zero ordinate value of the DTG curve.T/F
Thermal decomposition which overlap are often indicated more clearly by DTG curves than by the corresponding TG curves.T/F
Activity 2E:
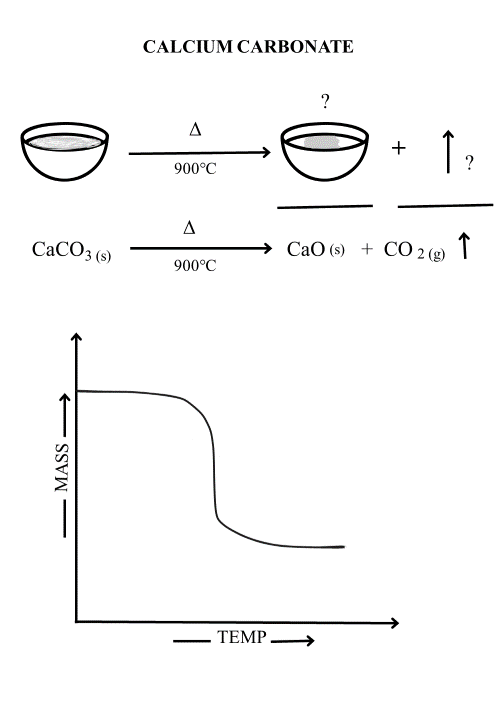
Activity 2F:
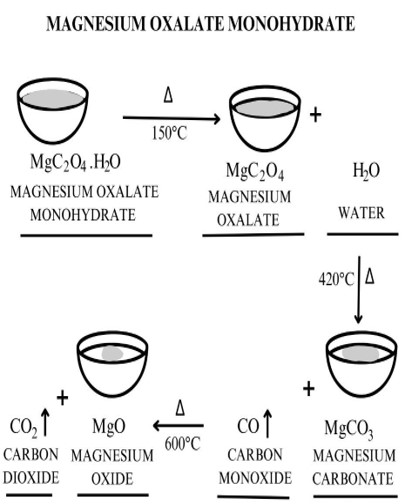
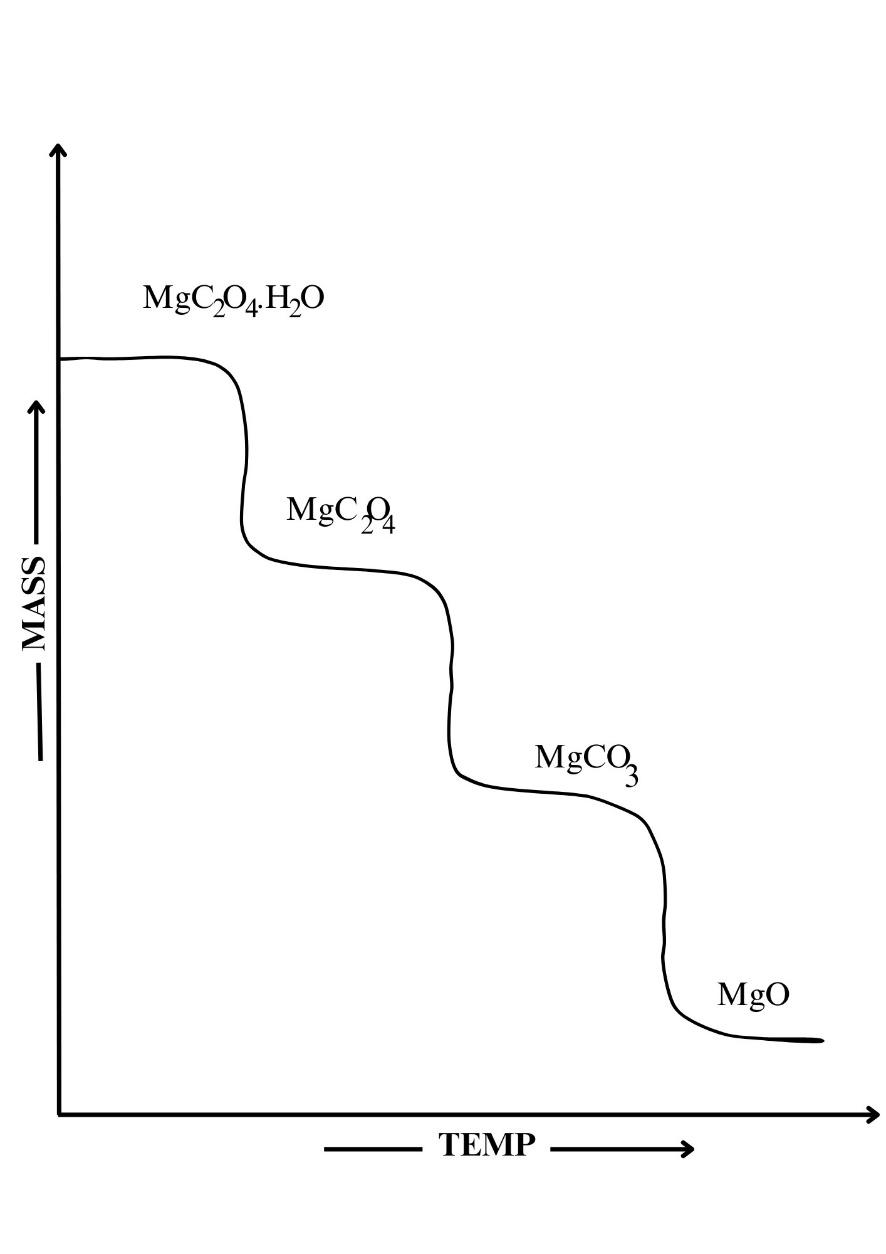
Activity 2G:
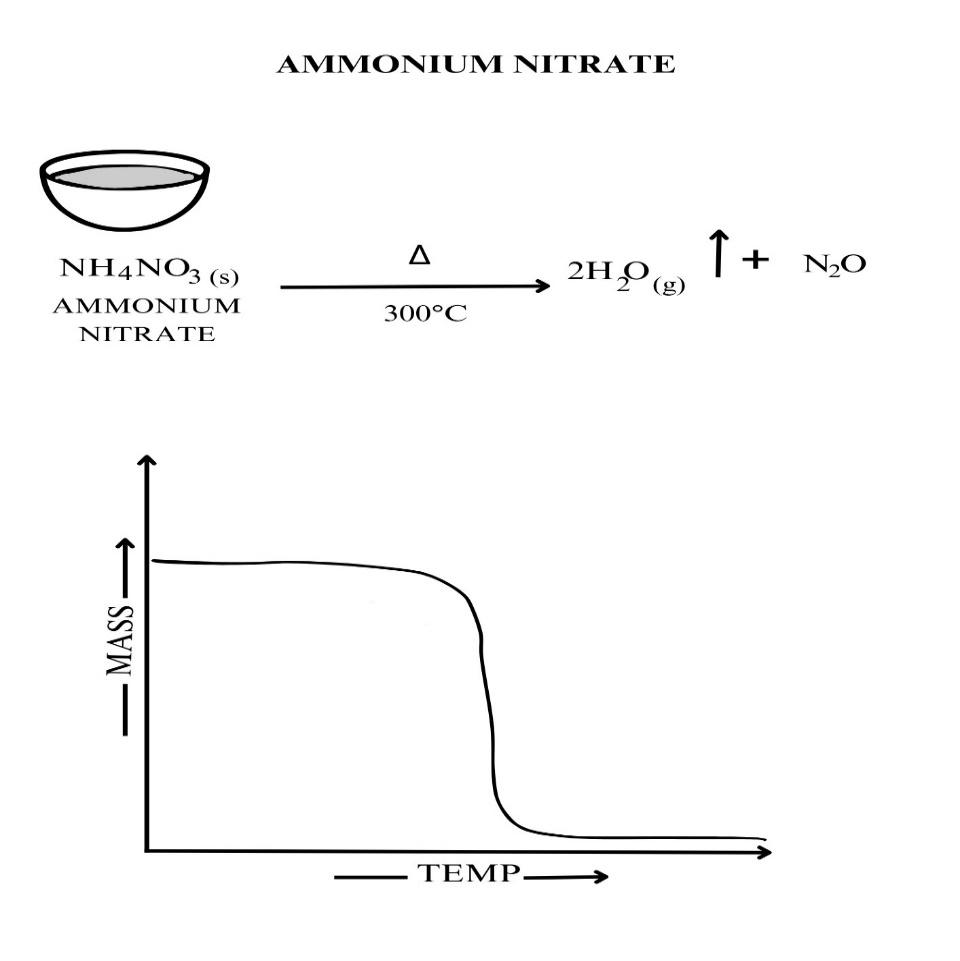
Activity 2H:
Activity: 2J
1. Ans: a
Ca(OH)2(s) →CaO (S) +H2O(g)
74.1g 56.1g 18g
% weight loss = 74.1-56.1/ 74.1 x 100 = 24.3%
Ans: b
6PbO(s) + O2(g) → 2Pb3O4(S)
1339.2 g 1371.2 g
% weight gain = 1371.2-1339.2/ 1339.2 x 100 = 2.4%
2. Ans:
The decrease in weight corresponds to the amount of carbon dioxide lost due to the decomposition of calcium carbonate present in the mixture as per the following reaction:
CaCO3(s)→ CaO (s) + CO2(g)↑
Weight loss = 250.6-190.8 = 59.8 mg
1mol of CaCO3 ≡ 1 mol of CO2
100.1mg of CaCO3≡ 44mg of CO2
? ≡ 59.8 mg of CO2
= 100.1 x 59.844/ 44 = 136.05 mg of CaCO3
Weight of the sample = 250.6 mg = mixture of CaCO3 & CaO
250.6 mg of mixture = 136.05 mg of CaCO3
100 mg of mixture = 136.05 x 100/ 250.6
= 54.29% of CaCO3
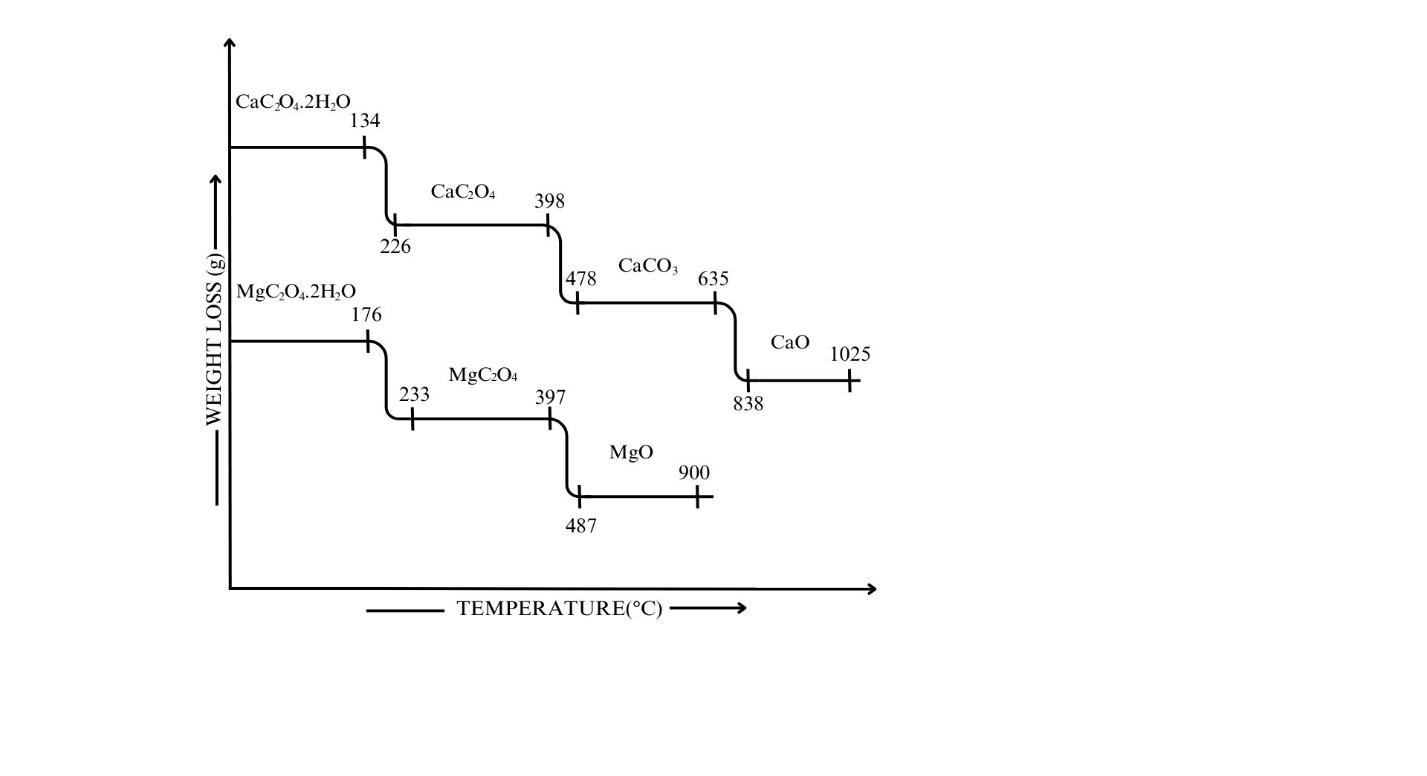
3.Ans: CaC2O4.H2O(s)→ CaC2O4(s) + H2O ↑
CaC2O4(s) → CaCO3(s) + CO(g) ↑
CaCO3(s) →CaO(s) + CO2(g) ↑
ii. Calculate the % weight loss at each step.
% weight loss = initial weight-final weight/ initial weight x 100
% weight loss at step 1 = 17.61-15.44/17.61×100
% weight loss at step 1 = 12.32
% weight loss at step 2 = 15.44-12.06/15.44 x 100
% weight loss at step 2= 21.89
% weight loss at step 3 = 12.06-6.76/ 12.06×100
% weight loss at step 3 = 43.95
Activity 3A:
The record shown is that of a DTA experiment since the ordinate plot ∆T which is a differential temperature. The downward direction of the peak indicates that a endothermic reaction has occurred. This in turn implies that the corresponding enthalpy change (∆H) must have been positive ie the value of enthalpy after the thermal effect was greater than its value before. This means that the sample took in heat during the reaction. Furthermore, there is evidence of a change in the heat capacity as the temperature is increased beyond the thermal transition. This is shown by the displacement of the baseline just beyond the end.
Answers: Select from the following list
[upward/downward, free energy/heat capacity, greater/less, DTG/DTA, base-line/background, derivative/differential, took in/gave out, negative/positive, enthalpy/entropy, before/after/during, exothermic/endothermic/isothermal, abscissa/ordinate, distortion/displacement.]
Activity 3B: Yes, they do offer an explanation. The curve for polyester shows that it melts at 255ᵒC. Molten polymer is in contact with the skin is a real hazard. Cotton, on the other hand, does not melt. It decomposes at 345ᵒC.
Activity 4A: Read each of the statements and mark them as True or False
(i) In DSC the sample and reference positions are provided with their own separate heating sources, so that the assembly may be operated on a ‘null balance’ basis. T
(ii) In DTA the equipment is so designed that the temperature of the sample is equal to that of the reference material at every point in the heating programme. F
(iii) Chemical decompositions which give rise to weight changes may be detected by DTA and DSC. T
(iv) The main components of a conventional differential thermal analyser consists of following: F (programmer missing)
a. The sample/reference holder
b. The thermocouple
c. The furnace
d. The amplifier
e. The recorder
Activity 4B:
If ∆H < 0, the system has undergone an endothermic/ exothermic change which means
Ts > TR [Choose the correct option: =, <, >]
Conversely ∆H > 0 means endothermic change and Ts < TR [Choose the correct option: =, <, >]
In order to keep ∆T = 0 [∆T = Ts – TR]
- In case of an endothermic reaction we must provide heat to sample/reference.
- In case of an exothermic reaction we must provide heat to sample/reference
Activity 4C: Observe the DSC curve given in ‘figure 4.3a.’ and answer the following
questions.
- How many transitions are seen in the above diagram? Ans: Two
- Do you see any endotherm or exotherm? If yes, how many? Ans: One endotherm and one exotherm.
- What does the endo or exothermic nature of the transition tell you about transition or ∆H value? Ans: Endotherm: + ve ∆H (heat is absorbed) Exotherm: -ve ∆H (heat is given out)
- Do you see a shift in the baseline post glass transition temperature? Ans: Yes, due to change in heat capacity
- Is the DSC curve of PET useful in predicting stability of the polymer? Justify your answer.
Yes, the melting point of any polymer is an important characteristic. It is the minimum temperature for processing the polymer and maximum temperature for using it. In addition, the glass transition temperature is useful since beyond this there is change in some of the physical properties of the polymer.
