Main Body
4. Differential Scanning Calorimetry (DSC)
 Learning Objectives
Learning Objectives
After studying the material in this Part, you should be able to:
- Explain the principle and working of DSC.
- Draw and interpret DSC curves.
- Compare and contrast DSC with TG and DTA.
- Explain the applications of DSC to polymeric materials and pharmaceuticals.
- Explain the applications of simultaneous TG-DTA-DSC analysis.
Differential scanning calorimetry (DSC) has become the most widely used thermal analysis technique. In this technique, the sample and the reference materials are subjected to a precisely programmed temperature change. DSC is very similar to DTA and gives much the same sort of information but DSC is more often used for quantitative measurement of energy changes.
4.1 Principle:
In DSC, the difference in temperature (∆T) between the sample and an inert reference is maintained at zero as they are subjected to controlled heating or cooling. The instrument is provided with a separate heater for the sample and the reference. When a thermal transition occurs in the sample, thermal energy is added to either the sample or the reference container in order to maintain both the sample and the reference at the same temperature. Because the energy transferred is exactly equivalent in magnitude to the energy absorbed or evolved in the transition, the balancing energy yields a direct calorimetric of the transition energy. Since DSC can measure directly both temperature and the enthalpy of a transition or the heat of a reaction, it is often substituted for differential thermal analysis as a means of determining these quantities except in certain high temperature applications.
4.2 Instrumentation and working:
A typical DSC cell uses a constantan (Cu-Ni) disk as the primary means of transferring heat to the sample and the reference positions and also as one element of the temperature-sensing thermoelectric junction. The sample and a reference are placed in separate pans that sit on raised platforms on the disk. Heat is transferred to the sample and reference through the disk. The differential heat flow to the sample and reference is monitored by the chromel/constantan thermocouples formed by the junction of the constantan disk and the chromel wafer covering the underside of each platform. Chromel and alumel wires connected to the underside of the wafers form a chromel/alumel thermocouple, which is used to directly monitor the sample temperature.
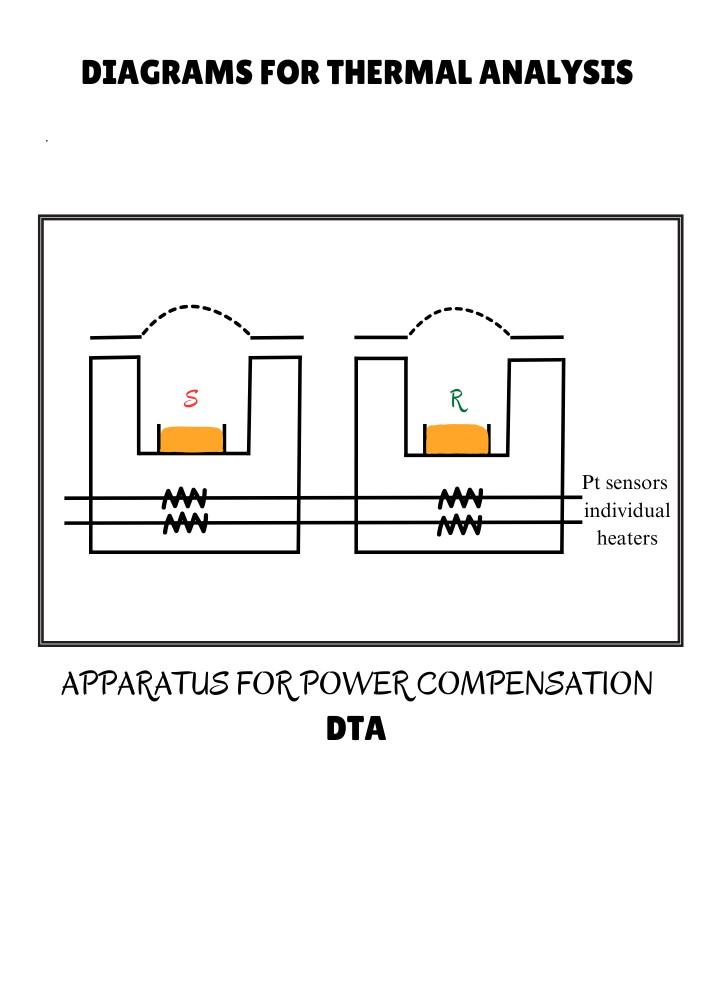 Figure 4.2a. DSC set up
Figure 4.2a. DSC set up
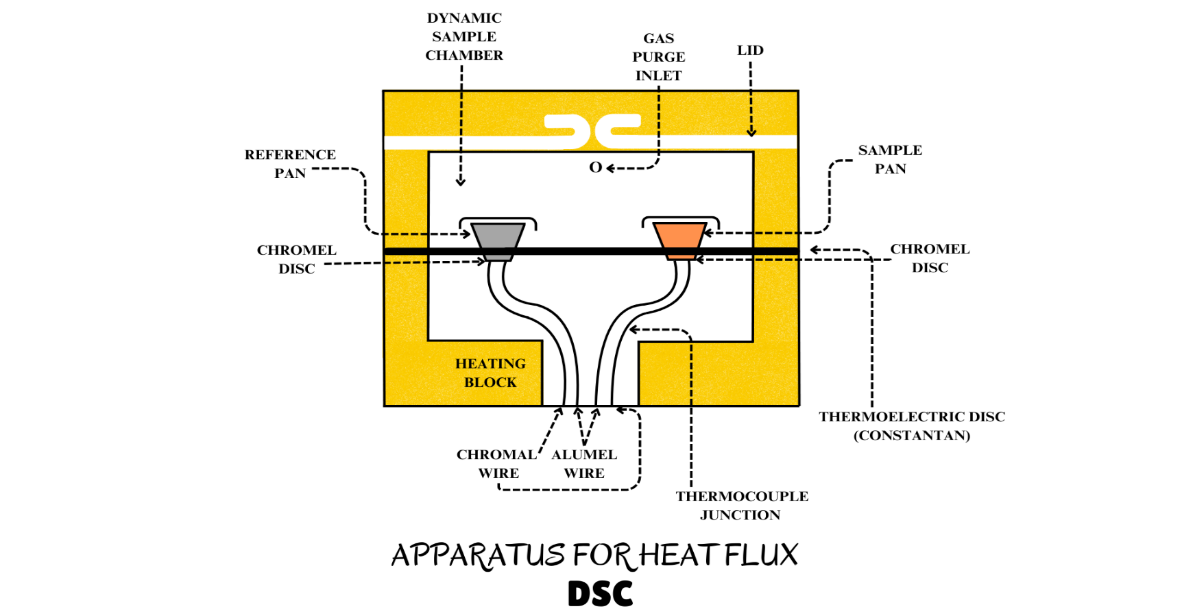
Figure 4.2b. DSC cell cross section
 Brain Teaser:
Brain Teaser:
Did you notice, in DTA there is a common heater and in DSC there is a separate heater for sample and the reference. Why?
 Activity 4A: Read each of the statements and mark them as True or False
Activity 4A: Read each of the statements and mark them as True or False
(i) In DSC the sample and reference positions are provided with their own separate
heating sources, so that the assembly may be operated on a ‘null balance’ basis.
(ii) In DTA the equipment is so designed that the temperature of the sample is equal
to that of the reference material at every point in the heating programme.
(iii) Chemical decompositions which give rise to weight changes may be detected by
DTA and DSC.
(iv) The main components of a conventional differential thermal analyser consist of following
a. The sample/reference holder
b. The thermocouple
c. The furnace
d. The amplifier
e. The recorder
4.3 DSC curves and its interpretation
The enthalpy of a sample refers to its heat content. Exothermic/Endothermic changes in a sample give rise to enthalpy changes. Enthalpy changes that may be taken to correspond to a heat of reaction are usually written as ∆H.
∆H= Hp – HR
Hp = Enthalpy of products
HR = Enthalpy of Reactants
A typical DSC curve is shown in Figure 4.3a
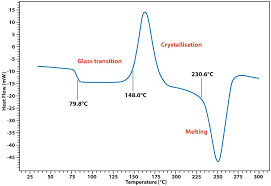
Figure 4.3a. DSC of PET
https://images.app.goo.gl/SUvdxf67c6U7NpgEA
 Activity 4B: Choose the correct option (James & Tonge, 2008)
Activity 4B: Choose the correct option (James & Tonge, 2008)
If ∆H < 0, the system has undergone an endothermic/ exothermic change which means
Ts __ TR [Choose the correct option: =, <, >]
Conversely ∆H > 0 means _______change and Ts ___ TR [Choose the correct option: =, <, >]
In order to keep ∆T = 0 [∆T = Ts – TR]
- In case of an endothermic reaction we must provide heat to sample/reference.
- In case of an exothermic reaction we must provide heat to sample/reference
 Activity 4C: Observe the DSC curve given in ‘figure 4.3a.’ and answer the following questions.
Activity 4C: Observe the DSC curve given in ‘figure 4.3a.’ and answer the following questions.
- How many transitions are seen in the above diagram?
- Do you see any endotherm or exotherm? If yes, how many?
- What does the endo or exothermic nature of the transition tell you about transition or ∆H value?
- Do you see a shift in the baseline post glass transition temperature?
- Is the DSC curve of PET useful in predicting stability of the polymer? Justify your answer.
In the above figure 4.3a, the shift in baseline is due to change in heat capacity (Cp) of the polymer post glass transition temperature.
Let us learn more about heat capacity, glass transition temperature and the role of DSC in characterisation of polymeric materials.
What does change in Cp means?
Heat Capacity (specific heat) is denoted as CP . It is the energy required to raise the temperature of one mole of material through one degree kelvin.
4.4 Applications of DSC:
I. Characterisation of polymeric materials: The ‘glass transition temperature’ (Tg) is an important parameter for many polymeric, ceramics and glasses. On cooling the material from the liquid state, there is (at the Tg) a change from the liquid state to amorphous state or glassy state. At this point there is discontinuity in the rate of change of the volume. There is also a change in the specific heat which allows study by DSC. Both these effects are illustrated in the figure 4.4a and 4.4b respectively.
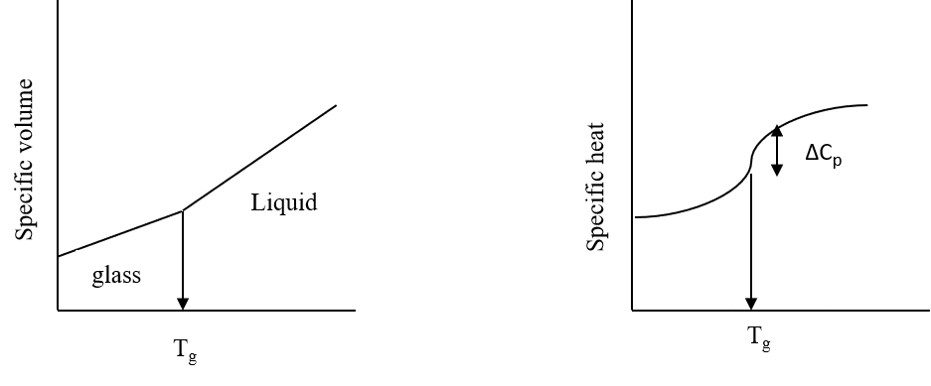
Figure 4.4a. Figure 4.4b.
II. Drug analysis for purity assessment: DSC provides rapid and reliable method for determining the purity of pharmaceuticals. The presence of trace impurities may reduce the effectiveness of a drug or even cause adverse effects. DSC technique allows the melting curve to be determined for the sample as it is heated through its melting point. Higher the impurity present in the sample, the lower its melting point and the broader its melting range.
Fig 4.4c shows comparative melting points of 98%, 99% and 100 mole % phenacetin. Since melting is an endothermic process but does not involve change in weight, it cannot be detected by TGA. DTA or DSC is the most suitable technique in such cases.
Pure compounds give sharp endothermic peak in DSC, which is evident from the peaks observed for 100% mole phenacetin. Impure compounds will melt at a temperature lower than the corresponding pure compounds. Hence, melting point and nature of peak can be used to comment on the purity of any drug.
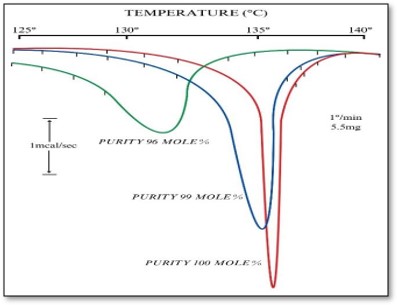
Figure 4.4c. DSC of Phenacetin
4.3 Comparison of DSC with DTA
|
|
DSC |
DTA |
|
1 |
It involves measurement of energy changes whilst the sample is subjected to controlled heating. |
It is a technique in which the difference in temperature between the sample and inert reference material, is measured as a function of temperature. |
|
2 |
It can detect all chemical and physical transitions including change in heat capacity. |
It can detect all physical and chemical transitions. |
|
3 |
It is a quantitative method. |
It is a semi-quantitative method. |
|
4 |
This technique is used to study purity of compounds, heat of reaction and characterization of polymers |
This technique is used to study phase transitions |
